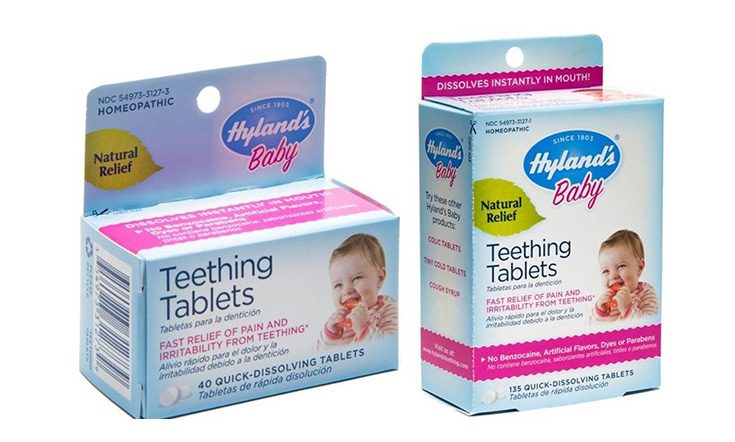
Recent concerns around the safety of homeopathic product, belladonna, has prompted the distributor of homeopathic company, Hyland’s, to recall their entire stock of teething tablets. A TGA announcement over the weekend announced that Hyland’s Baby Teething Tablets and Baby Night Time Teething Tablets should be returned to the place of purchase.
Hyland’s Australian distributor, Kadacs, was recalling all products as a safety precaution due to links with the deaths of babies in America. While products supplied in Australia have found to be safe, it is the unpredictable effects of belladonna which has caused alarm.
The TGA website states that “While the TGA had tested samples of these products supplied in Australia and found no quality issues, Kadac is now recalling the tablets as a precautionary measure due to the potential safety risk that belladonna alkaloids can pose to children. The effects of belladonna can be unpredictable and could cause serious health problems.”
It also notes that certain homeopathic teething products are not required to be on the Australian Register of Therapeutic Goods and are not assessed by the TGA when they are being imported in Australia. However, they are still bound by legislation outlined in theTherapeutic Goods Act 1989, which relates to safety and quality standards.
Hyland’s Baby Teething Gel is not affected by the recall, but all Hyland’s teething products (including the teething gel) will no longer be marketed in Australia.
If you have Hyland’s Baby teething tablets or Hyland’s Baby nighttime teething tablets you can return them to the place of purchase, or call the distributor, Kadac, on 1300 762 025. Do not continue to use the Hyland’s products.